Comparative Study of Antioxidant and Physicochemical Properties of Gracilaria fisheri Agar Obtained using Ultrasound-assisted Extraction without and with Enzymatic Treatment
Keywords:
Red Seaweed, Sulfated Agar, Enzymatic Treatment, Green Extraction, Antioxidant, Melting BehaviourAbstract
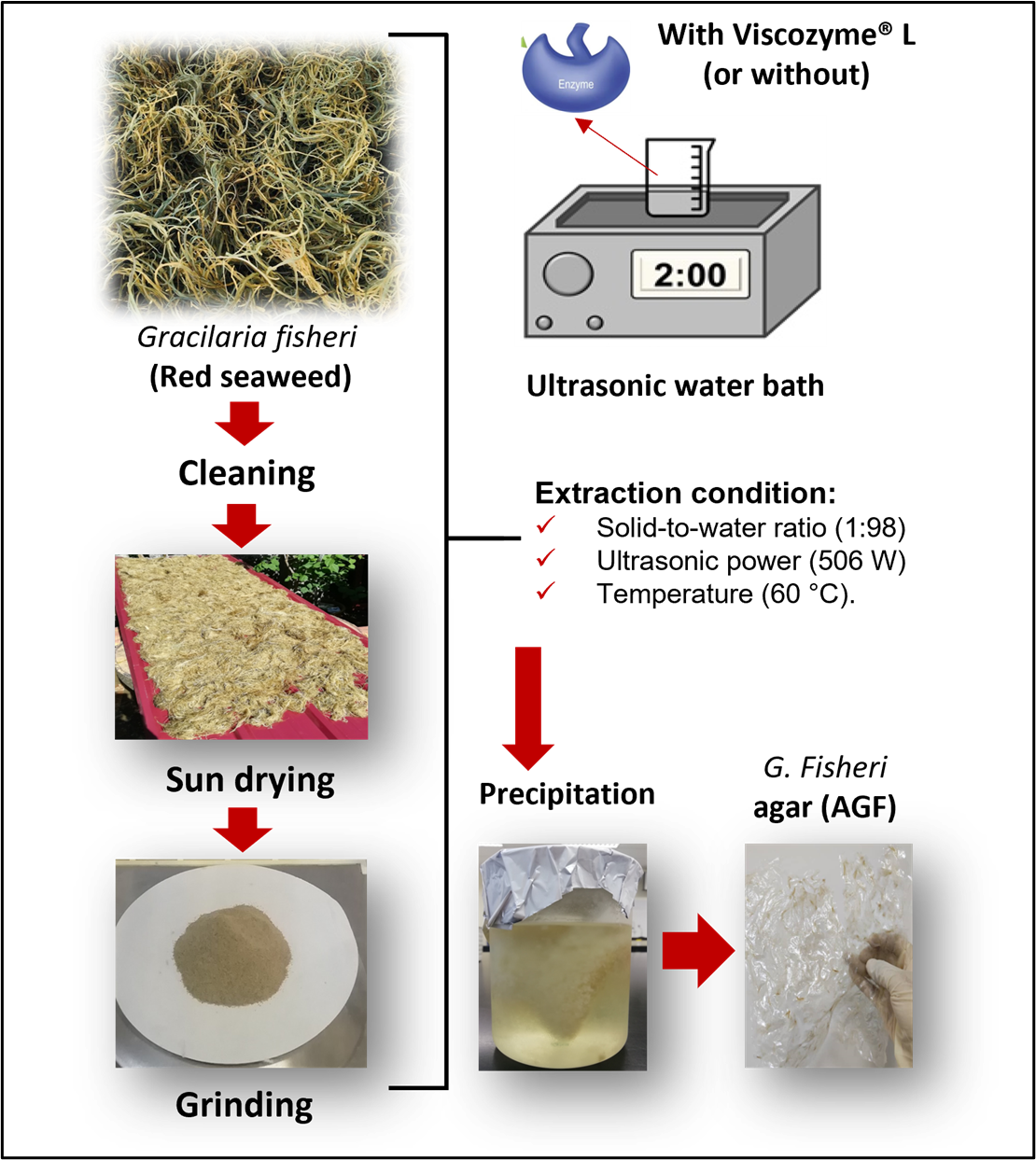
Ultrasound-assisted extraction (UAE) is increasingly utilised in agar extraction as an alternative to conventional methods. However, the UAE alone may not be sufficient to maximise the functional quality of agar. In this study, we compared the antioxidant and physicochemical properties of Gracilaria fisheri agar (AGF) extracted using UAE, without and with enzymatic treatment (0.006% Viscozyme® L), referred to as UAEE. The extraction was done using a solid-to-water ratio of 1:98, an ultrasonic power of 506 W, and a temperature of 60 ⁰C. The AGF from UAEE exhibited significantly (p < 0.05) lower IC50 value (0.13 mg/mL) than that from UAE (0.49 mg/mL), indicating a reduced amount of sample necessary to neutralise half of the 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radicals. Additionally, UAEE provided AGF with a significantly (p < 0.05) higher ascorbic acid equivalent antioxidant capacities (AAEC) of 11125.78 mg AA/100 g, compared to 2829.82 mg AA/100g for UAE. The enhanced antioxidant activity of AGF from UAEE may be attributed to its higher contents of total phenolic compounds, total sugars, and sulfate. Despite its higher sulfate content, it exhibited significantly (p < 0.05) higher melting properties, signifying its superior gelling properties over AGF from the UAE. However, it showed significantly (p < 0.05) lower ferric reducing antioxidant power, likely due to its lower galacturonic acid content relative to AGF from UAE. Overall, combining the UAE with enzymatic treatment resulted in a significantly higher AGF functional quality, offering a promising approach for the efficient and sustainable AGF extraction.
References
[1] S. Yarnpakdee, S. Benjakul, and P. Kingwascharapong, Physico-Chemical and Gel Properties of Agar from Gracilaria Tenuistipitata from the Lake of Songkhla, Thailand. Food Hydrocolloids 51 (2015) 217–226. https://doi.org/10.1016/j.foodhyd.2015.05.004.
[2] H.P.S. Abdul Khalil, T.K. Lai, Y.Y. Tye, S. Rizal, E.W.N. Chong, S.W. Yap, A.A. Hamzah, M.R. Nurul Fazita, and M.T. Paridah, A Review of Extractions of Seaweed Hydrocolloids: Properties and Applications. EXPRESS Polymer Letters 12 (2018) 296–317. https://doi.org/10.3144/expresspolymlett.2018.27.
[3] R.D. Villanueva, A.M.M. Sousa, M.P. Gonçalves, M. Nilsson, and L. Hilliou, Production and Properties of Agar from the Invasive Marine Alga, Gracilaria Vermiculophylla (Gracilariales, Rhodophyta). Journal of Applied Phycology 22 (2010) 211–220. https://doi.org/10.1007/s10811-009-9444-7.
[4] S. Imjongjairak, K. Ratanakhanokchai, N. Laohakunjit, C. Tachaapaikoon, P. Pason, and R. Waeonukul, Biochemical Characteristics and Antioxidant Activity of Crude and Purified Sulfated Polysaccharides from Gracilaria Fisheri. Bioscience, Biotechnology, and Biochemistry 80 (2016) 524–532. https://doi.org/10.1080/09168451.2015.1101334.
[5] E. Marinho-Soriano, and E. Bourret, Polysaccharides from the Red Seaweed Gracilaria Dura (Gracilariales, Rhodophyta). Bioresource Technology 96 (2005) 379–382. https://doi.org/10.1016/j.biortech.2004.04.012.
[6] J. Wang, Q. Zhang, Z. Zhang, J. Zhang, and P. Li, Synthesized Phosphorylated and Aminated Derivatives of Fucoidan and Their Potential Antioxidant Activity in Vitro. International Journal of Biological Macromolecules 44 (2009) 170–174. https://doi.org/10.1016/j.ijbiomac.2008.11.010.
[7] S.M. Hamed, S. Selim, G. Klöck, and H. AbdElgawad, Sensitivity of Two Green Microalgae to Copper Stress: Growth, Oxidative and Antioxidants Analyses. Ecotoxicology and Environmental Safety 144 (2017) 19–25. https://doi.org/10.1016/j.ecoenv.2017.05.048.
[8] M.P. Casas, E. Conde, H. Domínguez, and A. Moure, Ecofriendly Extraction of Bioactive Fractions from Sargassum Muticum. Process Biochemistry (Barking, London, England) 79 (2019) 166–173. https://doi.org/10.1016/j.procbio.2018.12.021.
[9] L.P. Gomez, C. Alvarez, M. Zhao, U. Tiwari, J. Curtin, M. Garcia-Vaquero, and B.K. Tiwari, Innovative Processing Strategies and Technologies to Obtain Hydrocolloids from Macroalgae for Food Applications. Carbohydrate Polymers 248 (2020) 116784. https://doi.org/10.1016/j.carbpol.2020.116784.
[10] N.N. Omar, N.H. Ibrahim, N.J. Mohamad, S.N. Achudan, and A. Mat Amin, Optimization of Ultrasound-Assisted Enzymatic Extraction Conditions on Yield, DPPH Antioxidant Activity, and Gel Strength of Agar from Gracilaria Fisheri. Journal of Aquatic Food Product Technology 33 (2024) 765–777. https://doi.org/10.1080/10498850.2024.2414432.
[11] V. Dewanto, X. Wu, K.K. Adom, and R.H. Liu, Thermal Processing Enhances the Nutritional Value of Tomatoes by Increasing Total Antioxidant Activity. Journal of Agricultural and Food Chemistry 50 (2002) 3010–3014. https://doi.org/10.1021/jf0115589.
[12] L.A. de la Rosa, E. Alvarez-Parrilla, and F. Shahidi, Phenolic Compounds and Antioxidant Activity of Kernels and Shells of Mexican Pecan (Carya Illinoinensis). Journal of Agricultural and Food Chemistry 59 (2011) 152–162. https://doi.org/10.1021/jf1034306.
[13] W. Brand-Williams, M.E. Cuvelier, and C. Berset, Use of a Free Radical Method to Evaluate Antioxidant Activity. Lebensmittel-Wissenschaft Und Technologie [Food Science and Technology] 28 (1995) 25–30. https://doi.org/10.1016/s0023-6438(95)80008-5.
[14] X.-J. Duan, W.-W. Zhang, X.-M. Li, and B.-G. Wang, Evaluation of Antioxidant Property of Extract and Fractions Obtained from a Red Alga, Polysiphonia Urceolata. Food Chemistry 95 (2006) 37–43. https://doi.org/10.1016/j.foodchem.2004.12.015.
[15] I.F. Benzie, and J.J. Strain, The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Analytical Biochemistry 239 (1996) 70–76. https://doi.org/10.1006/abio.1996.0292.
[16] K.S. Dodgson, and R.G. Price, A Note on the Determination of the Ester Sulphate Content of Sulphated Polysaccharides. The Biochemical Journal 84 (1962) 106–110. https://doi.org/10.1042/bj0840106.
[17] W.-K. Lee, Y.-Y. Lim, and C.-L. Ho, pH Affects Growth, Physiology and Agar Properties of Agarophyte Gracilaria Changii (Rhodophyta) under Low Light Intensity from Morib, Malaysia. Regional Studies in Marine Science 30 (2019) 100738. https://doi.org/10.1016/j.rsma.2019.100738.
[18] N. Peasura, N. Laohakunjit, O. Kerdchoechuen, and S. Wanlapa, Characteristics and Antioxidant of Ulva Intestinalis Sulphated Polysaccharides Extracted with Different Solvents. International Journal of Biological Macromolecules 81 (2015) 912–919. https://doi.org/10.1016/j.ijbiomac.2015.09.030.
[19] M. DuBois, K.A. Gilles, J.K. Hamilton, P.A. Rebers, and F. Smith, Colorimetric Method for Determination of Sugars and Related Substances. Analytical Chemistry 28 (1956) 350–356. https://doi.org/10.1021/ac60111a017.
[20] B.M. Khan, H.-M. Qiu, X.-F. Wang, Z.-Y. Liu, J.-Y. Zhang, Y.-J. Guo, W.-Z. Chen, Y. Liu, and K.-L. Cheong, Physicochemical Characterization of Gracilaria Chouae Sulfated Polysaccharides and Their Antioxidant Potential. International Journal of Biological Macromolecules 134 (2019) 255–261. https://doi.org/10.1016/j.ijbiomac.2019.05.055.
[21] S. Khongthong, Y. Theapparat, N. Roekngam, C. Tantisuwanno, M. Otto, and P. Piewngam, Characterization and Immunomodulatory Activity of Sulfated Galactan from the Red Seaweed Gracilaria Fisheri. International Journal of Biological Macromolecules 189 (2021) 705–714. https://doi.org/10.1016/j.ijbiomac.2021.08.182.
[22] V. Kumar, and R. Fotedar, Agar Extraction Process for Gracilaria Cliftonii (Withell, Millar, & Kraft, 1994). Carbohydrate Polymers 78 (2009) 813–819. https://doi.org/10.1016/j.carbpol.2009.07.001.
[23] W.-K. Lee, P. Namasivayam, and C.-L. Ho, Effects of Sulfate Starvation on Agar Polysaccharides of Gracilaria Species (Gracilariaceae, Rhodophyta) from Morib, Malaysia. Journal of Applied Phycology 26 (2014) 1791–1799. https://doi.org/10.1007/s10811-013-0231-0.
[24] H. Chen, F. Chen, Q. Xiao, M. Cai, Q. Yang, H. Weng, and A. Xiao, Structure and Physicochemical Properties of Amphiphilic Agar Modified with Octenyl Succinic Anhydride. Carbohydrate Polymers 251 (2021) 117031. https://doi.org/10.1016/j.carbpol.2020.117031.
[25] H. Chen, Q. Xiao, H. Weng, Y. Zhang, Q. Yang, and A. Xiao, Extraction of Sulfated Agar from Gracilaria Lemaneiformis Using Hydrogen Peroxide-Assisted Enzymatic Method. Carbohydrate Polymers 232 (2020) 115790. https://doi.org/10.1016/j.carbpol.2019.115790.
[26] E. de Sousa Oliveira Vanderlei, I.W.F. de Araújo, A.L.G. Quinderé, B.P. Fontes, Y.R.G. Eloy, J.A.G. Rodrigues, A.A.R. e Silva, H.V. Chaves, R.J.B. Jorge, D.B. de Menezes, J.S.A.M. Evangelista, M.M. Bezerra, and N.M.B. Benevides, The Involvement of the HO-1 Pathway in the Anti-Inflammatory Action of a Sulfated Polysaccharide Isolated from the Red Seaweed Gracilaria Birdiae. et al [Inflammation Research] 60 (2011) 1121–1130. https://doi.org/10.1007/s00011-011-0376-8.
[27] A.C.M. Leódido, L.E.C. Costa, T.S.L. Araújo, D.S. Costa, N.A. Sousa, L.K.M. Souza, F.B.M. Sousa, M.D.S. Filho, D.F.P. Vasconcelos, F.R.P. Silva, K.M. Nogueira, A.R. Araújo, F.C.N. Barros, A.L.P. Freitas, and J.V.R. Medeiros, Anti-Diarrhoeal Therapeutic Potential and Safety Assessment of Sulphated Polysaccharide Fraction from Gracilaria Intermedia Seaweed in Mice. International Journal of Biological Macromolecules 97 (2017) 34–45. https://doi.org/10.1016/j.ijbiomac.2017.01.006.
[28] S. Wu, M. Lu, and S. Wang, Amylase-Assisted Extraction and Antioxidant Activity of Polysaccharides from Gracilaria Lemaneiformis. 3 Biotech 7 (2017) 38. https://doi.org/10.1007/s13205-017-0697-6.
[29] S.F. K. Habeebullah, S. Alagarsamy, Z. Sattari, S. Al-Haddad, S. Fakhraldeen, A. Al-Ghunaim, and F. Al-Yamani, Enzyme-Assisted Extraction of Bioactive Compounds from Brown Seaweeds and Characterization. Journal of Applied Phycology 32 (2020) 615–629. https://doi.org/10.1007/s10811-019-01906-6.
[30] O. Gligor, A. Mocan, C. Moldovan, M. Locatelli, G. Crișan, and I.C.F.R. Ferreira, Enzyme-Assisted Extractions of Polyphenols – A Comprehensive Review. Trends in Food Science & Technology 88 (2019) 302–315. https://doi.org/10.1016/j.tifs.2019.03.029.
[31] M. Senevirathne, C.-B. Ahn, and J.-Y. Je, Enzymatic Extracts from Edible Red Algae, Porphyra Tenera, and Their Antioxidant, Anti-Acetylcholinesterase, and Anti-Inflammatory Activities. Food Science and Biotechnology 19 (2010) 1551–1557. https://doi.org/10.1007/s10068-010-0220-x.
[32] M. Sapatinha, A. Oliveira, S. Costa, S. Pedro, A. Gonçalves, R. Mendes, N.M. Bandarra, and C. Pires, Red and Brown Seaweeds Extracts: A Source of Biologically Active Compounds. Food Chemistry 393 (2022) 133453. https://doi.org/10.1016/j.foodchem.2022.133453.
[33] M. Monteiro, R.A. Santos, P. Iglesias, A. Couto, C.R. Serra, I. Gouvinhas, A. Barros, A. Oliva-Teles, P. Enes, and P. Díaz-Rosales, Effect of Extraction Method and Solvent System on the Phenolic Content and Antioxidant Activity of Selected Macro- and Microalgae Extracts. Journal of Applied Phycology 32 (2020) 349–362. https://doi.org/10.1007/s10811-019-01927-1.
[34] A. López, M. Rico, A. Rivero, and M. Suárez de Tangil, The Effects of Solvents on the Phenolic Contents and Antioxidant Activity of Stypocaulon Scoparium Algae Extracts. Food Chemistry 125 (2011) 1104–1109. https://doi.org/10.1016/j.foodchem.2010.09.101.
[35] J.Y. Wong, and F.Y. Chye, Antioxidant Properties of Selected Tropical Wild Edible Mushrooms. Journal of Food Composition and Analysis: An Official Publication of the United Nations University, International Network of Food Data Systems 22 (2009) 269–277. https://doi.org/10.1016/j.jfca.2008.11.021.
[36] M.M.V. Sasadara, and I.G.P. Wirawan, Effect of Extraction Solvent on Total Phenolic Content, Total Flavonoid Content, and Antioxidant Activity of Bulung Sangu (Gracilaria Sp.) Seaweed. IOP Conference Series. Earth and Environmental Science 712 (2021) 012005. https://doi.org/10.1088/1755-1315/712/1/012005.
[37] T.T. Dang, M.C. Bowyer, I.A. Van Altena, and C.J. Scarlett, Comparison of Chemical Profile and Antioxidant Properties of the Brown Algae. International Journal of Food Science & Technology 53 (2018) 174–181. https://doi.org/10.1111/ijfs.13571.
[38] A. Maadane, N. Merghoub, T. Ainane, H. El Arroussi, R. Benhima, S. Amzazi, Y. Bakri, and I. Wahby, Antioxidant Activity of Some Moroccan Marine Microalgae: Pufa Profiles, Carotenoids and Phenolic Content. Journal of Biotechnology 215 (2015) 13–19. https://doi.org/10.1016/j.jbiotec.2015.06.400.
[39] J.-R. Pang, V.M.-J. Goh, C.-Y. Tan, S.-M. Phang, K.-H. Wong, and Y.-Y. Yow, Neuritogenic and in Vitro Antioxidant Activities of Malaysian Gracilaria Manilaensis Yamamoto & Trono. Journal of Applied Phycology 30 (2018) 3253–3260. https://doi.org/10.1007/s10811-018-1438-x.
[40] H. Nagappan, P.P. Pee, S.H.Y. Kee, J.T. Ow, S.W. Yan, L.Y. Chew, and K.W. Kong, Malaysian Brown Seaweeds Sargassum Siliquosum and Sargassum Polycystum: Low Density Lipoprotein (LDL) Oxidation, Angiotensin Converting Enzyme (ACE), α-Amylase, and α-Glucosidase Inhibition Activities. Food Research International (Ottawa, Ont.) 99 (2017) 950–958. https://doi.org/10.1016/j.foodres.2017.01.023.
[41] A. Arulkumar, T. Rosemary, S. Paramasivam, and R.B. Rajendran, Phytochemical Composition, in Vitro Antioxidant, Antibacterial Potential and GC-MS Analysis of Red Seaweeds (Gracilaria Corticata and Gracilaria Edulis) from Palk Bay, India. Biocatalysis and Agricultural Biotechnology 15 (2018) 63–71. https://doi.org/10.1016/j.bcab.2018.05.008.
[42] M. Garcia-Vaquero, R. Ravindran, O. Walsh, J. O’Doherty, A.K. Jaiswal, B.K. Tiwari, and G. Rajauria, Evaluation of Ultrasound, Microwave, Ultrasound-Microwave, Hydrothermal and High Pressure Assisted Extraction Technologies for the Recovery of Phytochemicals and Antioxidants from Brown Macroalgae. Marine Drugs 19 (2021) 309. https://doi.org/10.3390/md19060309.
[43] S. Cox, N. Abu-Ghannam, and S. Gupta, An Assessment of the Antioxidant and Antimicrobial Activity of Six Species of Edible Irish Seaweeds. (2010). https://doi.org/10.21427/D7HC92.
[44] S.M. Mohy El-Din, and A.M.D. El-Ahwany, Bioactivity and Phytochemical Constituents of Marine Red Seaweeds (Jania Rubens,Corallina MediterraneaandPterocladia Capillacea). Journal of Taibah University for Science: JTUSCI 10 (2016) 471–484. https://doi.org/10.1016/j.jtusci.2015.06.004.
[45] B. Matthäus, Antioxidant Activity of Extracts Obtained from Residues of Different Oilseeds. Journal of Agricultural and Food Chemistry 50 (2002) 3444–3452. https://doi.org/10.1021/jf011440s.
[46] Y. Athukorala, K.-N. Kim, and Y.-J. Jeon, Antiproliferative and Antioxidant Properties of an Enzymatic Hydrolysate from Brown Alga, Ecklonia Cava. Food and Chemical Toxicology: An International Journal Published for the British Industrial Biological Research Association 44 (2006) 1065–1074. https://doi.org/10.1016/j.fct.2006.01.011.
[47] K.P. Devi, N. Suganthy, P. Kesika, and S.K. Pandian, Bioprotective Properties of Seaweeds: In Vitro Evaluation of Antioxidant Activity and Antimicrobial Activity against Food Borne Bacteria in Relation to Polyphenolic Content. BMC Complementary and Alternative Medicine 8 (2008) 38. https://doi.org/10.1186/1472-6882-8-38.
[48] Y.L. Chew, Y.Y. Lim, M. Omar, and K.S. Khoo, Antioxidant Activity of Three Edible Seaweeds from Two Areas in South East Asia. Lebensmittel-Wissenschaft Und Technologie [Food Science and Technology] 41 (2008) 1067–1072. https://doi.org/10.1016/j.lwt.2007.06.013.
[49] J. Wang, H. Guo, J. Zhang, X. Wang, B. Zhao, J. Yao, and Y. Wang, Sulfated Modification, Characterization and Structure–Antioxidant Relationships of Artemisia Sphaerocephala Polysaccharides. Carbohydrate Polymers 81 (2010) 897–905. https://doi.org/10.1016/j.carbpol.2010.04.002.
[50] S.U. Kadam, C. Álvarez, B.K. Tiwari, and C.P. O’Donnell, Extraction of biomolecules from seaweeds, in: Seaweed Sustainability, Elsevier, 2015: pp. 243–269. https://doi.org/10.1016/b978-0-12-418697-2.00009-x.
[51] P.T. Chan, P. Matanjun, S. Yasir, and T.S. Tan, Antioxidant Activities and Polyphenolics of Various Solvent Extracts of Red Seaweed, Gracilaria Changii. Journal of Applied Phycology 27 (2015) 2377–2386. https://doi.org/10.1007/s10811-014-0493-1.
[52] V. Steenkamp, N. O., J. van Tonder, A. Dinsmore, and M. Gulumian, Evaluation of the Phenolic and Flavonoid Contents and Radical Scavenging Activity of Three Southern African Medicinal Plants. African Journal of Pharmacy and Pharmacology 7 (2013) 703–709.
[53] M. Francavilla, M. Franchi, M. Monteleone, and C. Caroppo, The Red Seaweed Gracilaria Gracilis as a Multi Products Source. Marine Drugs 11 (2013) 3754–3776. https://doi.org/10.3390/md11103754.
[54] A.M.M. Sousa, V.D. Alves, S. Morais, C. Delerue-Matos, and M.P. Gonçalves, Agar Extraction from Integrated Multitrophic Aquacultured Gracilaria Vermiculophylla: Evaluation of a Microwave-Assisted Process Using Response Surface Methodology. Bioresource Technology 101 (2010) 3258–3267. https://doi.org/10.1016/j.biortech.2009.12.061.
[55] Q. Xiao, H. Weng, H. Ni, Q. Hong, K. Lin, and A. Xiao, Physicochemical and Gel Properties of Agar Extracted by Enzyme and Enzyme-Assisted Methods. Food Hydrocolloids 87 (2019) 530–540. https://doi.org/10.1016/j.foodhyd.2018.08.041.
[56] T. Wang, R. Jónsdóttir, H.G. Kristinsson, G.O. Hreggvidsson, J.Ó. Jónsson, G. Thorkelsson, and G. Ólafsdóttir, Enzyme-Enhanced Extraction of Antioxidant Ingredients from Red Algae Palmaria Palmata. Lebensmittel-Wissenschaft Und Technologie [Food Science and Technology] 43 (2010) 1387–1393. https://doi.org/10.1016/j.lwt.2010.05.010.
[57] B.Y.N. Arroyo, S.L.O. Chacón, V.G. Tacias-Pascacio, G.E.O. Chacón, C.V. Canseco, R.M. Gordillo, and A.R. Quintero, Aqueous Enzymatic Extraction of Oil from Microwave-Pretreated Jicaro Seeds. Current Biochemical Engineering 5 (2019) 42–49. https://doi.org/10.2174/2212711906666190131150922.
[58] M.D.N. Meinita, B. Marhaeni, Y.-K. Hong, and G.-T. Jeong, Enzymatic Saccharification of Agar Waste from Gracilaria Verrucosa and Gelidium Latifolium for Bioethanol Production. Journal of Applied Phycology 29 (2017) 3201–3209. https://doi.org/10.1007/s10811-017-1205-4.
[59] E. Trigueros, M.T. Sanz, A. Filipigh, S. Beltrán, and P. Riaño, Enzymatic Hydrolysis of the Industrial Solid Residue of Red Seaweed after Agar Extraction: Extracts Characterization and Modelling. Food and Bioproducts Processing 126 (2021) 356–366. https://doi.org/10.1016/j.fbp.2021.01.014.
[60] H. Essa, D. Fleita, D. Rifaat, S. Samy, and M. El-Sayed, Towards Optimizing the Conventional and Ultrasonic-Assisted Extraction of Sulfated Polysaccharides from Marine Algae. IOP Conference Series. Materials Science and Engineering 464 (2018) 012007. https://doi.org/10.1088/1757-899x/464/1/012007.
[61] M. Jridi, R. Nasri, Z. Marzougui, O. Abdelhedi, M. Hamdi, and M. Nasri, Characterization and Assessment of Antioxidant and Antibacterial Activities of Sulfated Polysaccharides Extracted from Cuttlefish Skin and Muscle. International Journal of Biological Macromolecules 123 (2019) 1221–1228. https://doi.org/10.1016/j.ijbiomac.2018.11.170.
[62] S. Chang, X. Zou, B. Zhu, L. You, Z. Zhao, and K. Hileuskaya, The Characteristics of Polysaccharide from Gracilaria Chouae and Its Application in Food Packaging with Carboxymethyl Cellulose and Lysozyme. Food Hydrocolloids 135 (2023) 108109. https://doi.org/10.1016/j.foodhyd.2022.108109.
[63] H. Tian, H. Liu, W. Song, L. Zhu, T. Zhang, R. Li, and X. Yin, Structure, Antioxidant and Immunostimulatory Activities of the Polysaccharides from Sargassum Carpophyllum. Algal Research 49 (2020) 101853. https://doi.org/10.1016/j.algal.2020.101853.
[64] C.M.R. Rocha, A.M.M. Sousa, J.K. Kim, J.M.C.S. Magalhães, C. Yarish, and M. do P. Gonçalves, Characterization of Agar from Gracilaria Tikvahiae Cultivated for Nutrient Bioextraction in Open Water Farms. Food Hydrocolloids 89 (2019) 260–271. https://doi.org/10.1016/j.foodhyd.2018.10.048.
[65] J.S. Gomes-Dias, S.G. Pereira, J.A. Teixeira, and C.M.R. Rocha, Hydrothermal Treatments – A Quick and Efficient Alternative for Agar Extraction from Gelidium Sesquipedale. Food Hydrocolloids 132 (2022) 107898. https://doi.org/10.1016/j.foodhyd.2022.107898.
[66] L.P. Gómez Barrio, E.M. Cabral, M. Zhao, C. Álvarez García, R. Senthamaraikannan, R.B. Padamati, U. Tiwari, J.F. Curtin, and B.K. Tiwari, Comparison Study of an Optimized Ultrasound-Based Method versus an Optimized Conventional Method for Agar Extraction, and Protein Co-Extraction, from Gelidium Sesquipedale. Foods (Basel, Switzerland) 11 (2022) 805. https://doi.org/10.3390/foods11060805.
[67] G. Archana, K. Sabina, S. Babuskin, K. Radhakrishnan, M.A. Fayidh, P.A.S. Babu, M. Sivarajan, and M. Sukumar, Preparation and Characterization of Mucilage Polysaccharide for Biomedical Applications. Carbohydrate Polymers 98 (2013) 89–94. https://doi.org/10.1016/j.carbpol.2013.04.062.
[68] A.R. Ganesan, M. Shanmugam, S. Palaniappan, and G. Rajauria, Development of Edible Film from Acanthophora Spicifera : Structural, Rheological and Functional Properties. Food Bioscience 23 (2018) 121–128. https://doi.org/10.1016/j.fbio.2017.12.009.
[69] B.W.S. Souza, M.A. Cerqueira, A.I. Bourbon, A.C. Pinheiro, J.T. Martins, J.A. Teixeira, M.A. Coimbra, and A.A. Vicente, Chemical Characterization and Antioxidant Activity of Sulfated Polysaccharide from the Red Seaweed Gracilaria Birdiae. Food Hydrocolloids 27 (2012) 287–292. https://doi.org/10.1016/j.foodhyd.2011.10.005.
[70] Y. Gong, Y. Ma, P.C.-K. Cheung, L. You, L. Liao, S. Pedisić, and V. Kulikouskaya, Structural Characteristics and Anti-Inflammatory Activity of UV/H2O2-Treated Algal Sulfated Polysaccharide from Gracilaria Lemaneiformis. Food and Chemical Toxicology: An International Journal Published for the British Industrial Biological Research Association 152 (2021) 112157. https://doi.org/10.1016/j.fct.2021.112157.
Downloads
Published
Issue
Section
License
Copyright (c) 2025 Progress in Energy and Environment

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.











