Influence of Glucose on the Microstructure of Electrodeposited Nickel-Al2O3 Nanoparticles and the Corrosion Resistance of Low-carbon Steel
Keywords:
Electro-deposition, Steel, Nickel, Glucose, Microstructure, Corrosion BehaviorAbstract
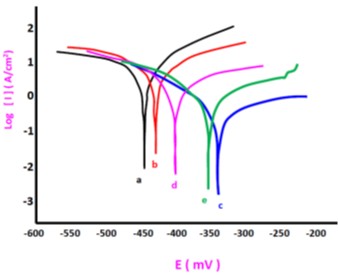
The purpose of coating steel surfaces is to enhance their properties, a challenge that continues to attract scientific interest. Selecting an appropriate coating involves choosing the most suitable method and optimizing process conditions to achieve the desired surface characteristics. Within this context, the present study investigates the effect of glucose on the microstructure of nickel coatings electrodeposited on low-carbon steel in the presence of Al₂O₃ nanoparticles. Glucose was selected as an alternative to saccharin, a commonly used additive in nickel electroplating. The coating was applied using electro-deposition in a Watts bath, and its structural characteristics were analyzed using scanning electron microscopy (SEM) and X-ray diffraction (XRD). Corrosion resistance was evaluated via voltammetric measurements in a 3.5% NaCl solution. The results revealed that nickel particles formed along a preferred crystallographic orientation, and the incorporation of glucose altered the particle morphology, leading to the formation of pyramid-shaped structures at a concentration of 2 g/L. Furthermore, the addition of glucose significantly enhanced the corrosion resistance of the coating, achieving a maximum polarization resistance (Rp) of 43.86 kΩ·cm².